29 Chapter 29: Respiration: ETC and Oxidative Phosphorylation
Lisa Limeri
Learning Objectives
By the end of this section, students will be able to:
- Model aerobic cellular respiration, including glycolysis, pyruvate processing, the citric acid cycle, and oxidative phosphorylation, in terms of the following inputs and outputs: NADH, FADH₂, Glucose, Acetyl CoA, Pyruvate, O₂, CO₂, H⁺ gradients, and ATP.
- Draw a model depicting the flow of electrons through molecules and the changes in their energy levels through cellular respiration
- Predict the effects of altering specific parts of the electron transport chain or ATP synthase.
Introduction
You have just read about two pathways in glucose catabolism—glycolysis and the citric acid cycle—that generate ATP. Most of the ATP generated during the aerobic catabolism of glucose, however, is not generated directly from these pathways. Instead, it is derived from a process that begins by moving electrons through a series of electron carriers that undergo redox reactions. This process causes hydrogen ions to accumulate within the intermembrane space in the mitochondrion. Therefore, a concentration gradient forms in which hydrogen ions diffuse out of the intermembrane space into the mitochondrial matrix by passing through a protein called ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP. The vast majority of ATP produced through aerobic cellular respiration is created by ATP synthase, harvesting the power of the hydrogen ion concentration gradient across the inner mitochondrial membrane.
Electron Transport Chain (ETC)
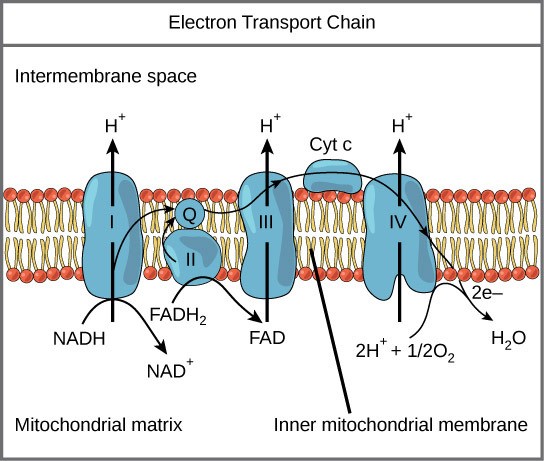
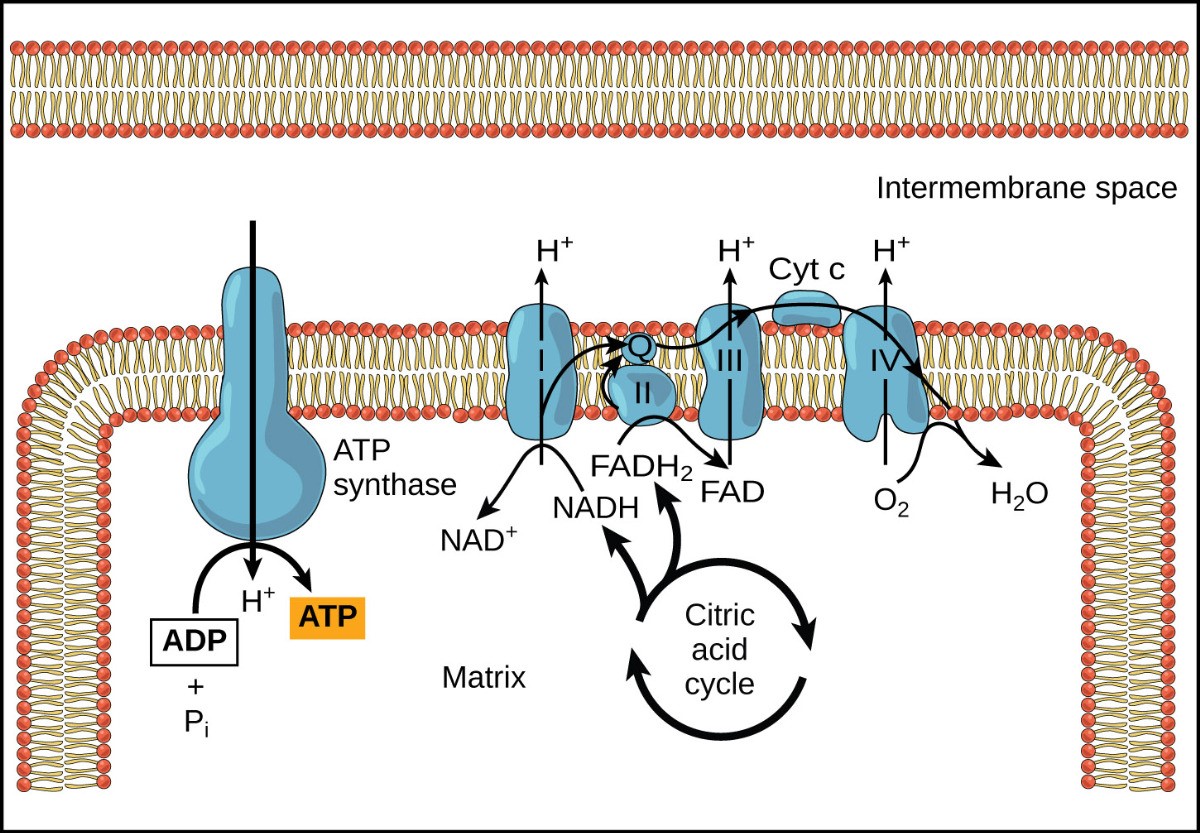
The electron transport chain (Figure 28.1) is the only part of glucose metabolism that directly uses oxygen. During the electron transport chain, electrons are passed through four complexes of proteins, dropping to lower energy states with each move. This energy is harnessed to pump proton (H+ ions) across the inner mitochondrial membrane to create a high H+ concentration inside of the inter-membrane space of the mitochondrion. Oxygen has high electronegativity and can accept very low-energy electrons, allowing the cell to extract a large amount of energy from passing electrons to lower energy states before finally passing them to oxygen. Electron transport is a series of redox reactions in which electrons are passed rapidly from one complex of proteins to the next. Electrons enter the electron transport chain from the high-energy electron carriers (NADH and FADH2) that were produced during glycolysis, pyruvate processing, and the citric acid cycle. There are 4 components that make up the electron transport chain, each of which are a complex of proteins embedded in the inner mitochondrial membrane (Fig 28.1). Carrier molecules, Q and Cytochrome C shuttle electrons through the complexes (Fig 28.1). At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium, and water is formed. Thus, oxygen is the final electron acceptor in the electron transport chain.
Reading Question #1
The electron transport chain complexes are located in…
A. the cytoplasm of the cell.
B. the mitochondrial inter-membrane space.
C. the mitochondrial matrix.
D. the inner mitochondrial membrane.
Complex I
First, two electrons are carried to the first complex via NADH. This complex, labeled I, is composed of flavin mononucleotide (FMN) and an iron-sulfur (Fe-S)-containing protein. FMN, which is derived from vitamin B2 (also called riboflavin), is one of several prosthetic groups or cofactors in the electron transport chain. A prosthetic group is a nonprotein molecule required for the activity of a protein. Prosthetic groups are organic or inorganic, nonpeptide molecules bound to a protein that facilitates its function. Prosthetic groups include coenzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase and is a very large protein, containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space, contributing to establishing the hydrogen ion gradient across the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives electrons from FADH2—which does not pass its electrons to complex I. The compound connecting the first and second complexes to the third is ubiquinone (called Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from complex I, and the electrons derived from FADH2 from complex II. Q and FADH2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH2 electrons. The number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third complex is composed of cytochrome b—another Fe-S protein, a Rieske center (2Fe-2S center), and cytochrome c proteins. This complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe2+ (reduced) and Fe3+ (oxidized). The heme molecules in the cytochromes have slightly different characteristics due to the effects of the different proteins binding to them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes.
Complex IV
The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced by the gain of two electrons. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H2O). The removal of the hydrogen ions from the system contributes to the ion gradient that forms the foundation for the process of chemiosmosis.
Reading Question #2
The energy that is released by electrons moving through the electron transport chain is used to do what?
A. Directly phosphorylate ADP to generate ATP.
B. Create a hydrogen ion electrochemical gradient.
C. Oxidize NAD+ and FADH2.
D. Oxidize water.
Chemiosmosis
The electron transport chain set up a high concentration of hydrogen ions (protons) within the inter-membrane space, which means there is a strong electrochemical gradient across the inner mitochondrial membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions’ positive charge and their aggregation on one side of the membrane. Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient.
If the membrane were continuously open to simple diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Recall that ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Hydrogen ions in the intermembrane space can only pass through the inner mitochondrial membrane by an integral membrane protein called ATP synthase (Figure 28.2). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion gradient.
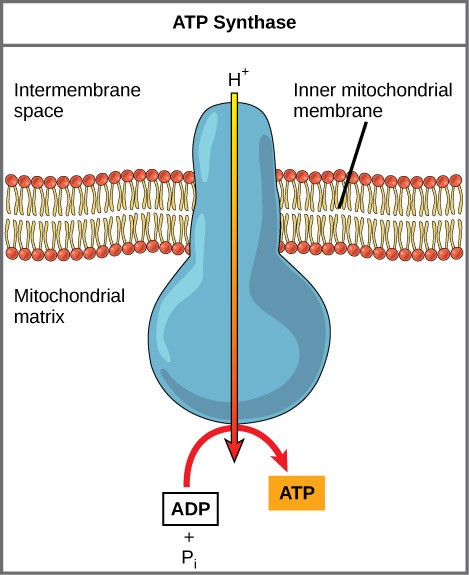
Chemiosmosis is used to generate 90% of the ATP made during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight to create ATP. The production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall result of these reactions is the production of ATP from the energy harvested from moving electrons. These electrons were originally part of a glucose molecule.
Reading Question #3
What is the original source and final destination of electrons during cellular respiration (starting with glycolysis and ending with oxidative phosphorylation)?
A. Source is oxygen; destination is oxygen.
B. Source is glucose; destination is carbon dioxide.
C. Source is glucose; destination is water.
D. Source is water; destination is glucose.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria (The NADH generated from glycolysis cannot easily enter mitochondria). Thus, electrons are picked up on the inside of mitochondria by either NAD+ or FAD. As you have learned earlier, these FAD molecules can transport lower-energy electrons; consequently, fewer ATP molecules are generated when FAD acts as a carrier.
Another factor that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are also used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For example, sugars other than glucose are fed into the glycolytic pathway for energy extraction. In addition, the five-carbon sugars that form nucleic acids are made from intermediates in glycolysis. Certain nonessential amino acids can be made from intermediates of both glycolysis and the citric acid cycle. Lipids, such as cholesterol and triglycerides, are also made from intermediates in these pathways, and both amino acids and triglycerides are broken down for energy through these pathways. Overall, in living systems, these pathways of glucose catabolism extract about 34% of the energy contained in glucose, with the remainder being released as heat.
Reading Question #4
Oxidative phosphorylation is the process by which cells…
A. generate ATP in the absence of oxygen.
B. generate simple sugars.
C. conduct redox reactions.
D. harness an electrochemical gradient to generate ATP.
Reading Question #5
The vast majority of ATP produced during cellular respiration is produced during which step?
A. Glycolysis
B. Fermentation
C. Citric Acid Cycle
D. Oxidative phosphorylation
Acknowledgements
Adapted from Clark, M.A., Douglas, M., and Choi, J. (2018,). Biology 2e. OpenStax. Retrieved from https://openstax.org/books/biology-2e/pages/1-introduction

